Blood Pressure Medication Recall : What Patients Need to Know About the Latest FDA Action

In a significant move to protect public health, U.S. drug manufacturers have recalled more than half a million bottles of a common blood pressure medication due to potential contamination with a cancer-causing chemical. The voluntary recall, initiated by Teva Pharmaceuticals USA and distributor Amerisource Health Services, highlights the ongoing challenge of ensuring the purity of the nation’s drug supply .
For the millions of Americans managing hypertension, this blood pressure medication recall serves as a critical reminder to stay informed about their prescriptions. The affected drug, prazosin hydrochloride, is also used to treat nightmares associated with post-traumatic stress disorder (PTSD), widening the circle of patients who need to check their medicine cabinets .
What Was Recalled? The Specific Products Affected
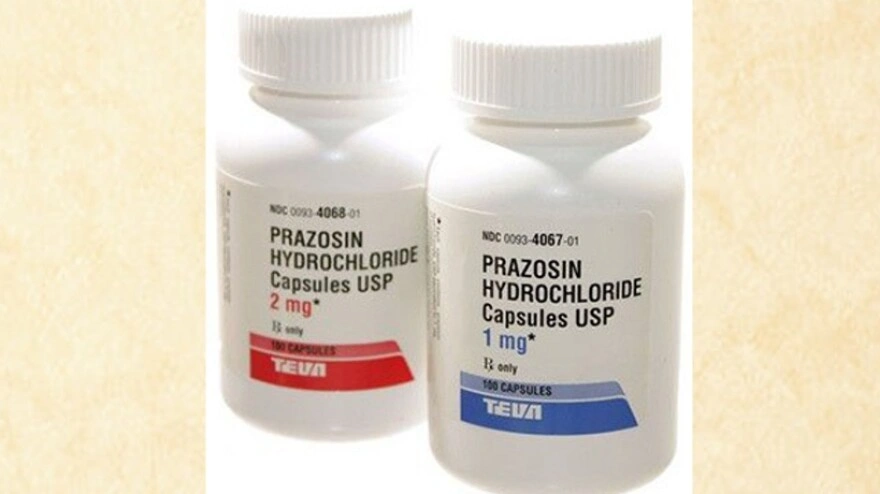
The recall targets specific lots of prazosin hydrochloride capsules. The U.S. Food and Drug Administration (FDA) announced that the recall involves a substantial 580,000 bottles of the medication across three different dosage strengths .
The table below breaks down the specific products included in this blood pressure medication recall:
The recalled bottles were distributed nationwide and packaged in quantities of 100, 500, and 1,000 capsules . The FDA has classified this as a Class II recall, meaning that while exposure to the affected product may cause temporary or medically reversible health consequences, the probability of serious adverse health effects is remote .
Why Was This Blood Pressure Medication Recalled?
The recall was initiated after laboratory testing revealed elevated levels of a chemical impurity called N-nitroso prazosin impurity C . This compound belongs to a class of chemicals known as nitrosamines.
Understanding Nitrosamine Impurities
Nitrosamines are common environmental contaminants found in some drinking water and foods, including cured and grilled meats and dairy products. Everyone is exposed to trace amounts of them .
Regarding drug safety, the FDA explains that these impurities can sometimes form during a drug’s chemical manufacturing process or during its storage . While the agency permits minute, acceptable levels of certain nitrosamines in pharmaceuticals, the concentration found in these recalled lots of prazosin was above the established acceptable intake limit .
The Associated Cancer Risk
The primary concern with nitrosamine impurities is their potential to be carcinogenic, or cancer-causing, particularly when people are exposed to them above acceptable levels over long periods .
It is crucial to understand the context of this risk. The FDA states that a person taking a drug with nitrosamine levels at or below the acceptable daily intake limit every day for 70 years would not be expected to have an increased risk of cancer . The recall was initiated because the levels in these specific lots exceeded that safety threshold, prompting action from the manufacturer and regulators to protect patients from prolonged exposure .
Critical Guidance for Patients: What You Should Do Now
If you are currently taking prazosin hydrochloride, do not panic. Medical experts and regulatory authorities provide clear steps to ensure your safety.
- ✔️ Do Not Stop Taking Your Medication: The most important instruction from the FDA is to continue taking your current medicine until your pharmacist provides a replacement or your doctor prescribes an alternative . Abruptly stopping blood pressure medication can lead to a dangerous spike in blood pressure, which poses a more immediate health risk than the potential long-term risk from the impurity .
- ✔️ Check Your Prescription: Look at the prescription bottle to identify the drug name, dosage strength, and manufacturer (Teva Pharmaceuticals). You can then check the specific lot number on your bottle against the list on the FDA’s Enforcement Reports page to see if your medication is part of the recall .
- ✔️ Consult Your Doctor or Pharmacist: If you find that your medication is part of the recalled lots, or if you are unsure, contact your healthcare provider or pharmacy immediately . They can advise you on the next steps, which will likely involve replacing your current prescription with a batch that is not affected.
To date, Teva Pharmaceuticals has not received any reports of adverse events linked to this impurity in the recalled products .
A Recurring Challenge in the Pharmaceutical Industry
This blood pressure medication recall is part of a broader, ongoing effort by the FDA and global regulators to detect and remove nitrosamine impurities from the drug supply. In recent years, similar recalls have affected other classes of blood pressure drugs, including certain valsartan, losartan, and irbesartan medications .
The FDA continues to work with drug manufacturers to refine production processes and implement more rigorous testing to prevent these impurities from forming . For patients, these recalls underscore the importance of a robust regulatory system and the need for vigilance, but they should also provide confidence that safeguards are in place to identify and remove potentially harmful products from the market.
Staying Informed and Proactive About Your Health
Staying informed about recalls is a key part of managing your health. Patients and caregivers can regularly check the FDA’s drug recall webpage for the latest safety announcements . Always communicate openly with your healthcare provider about any concerns regarding your medications, and never discontinue a prescribed treatment without first seeking professional medical advice.
This latest action on prazosin demonstrates the protective checks and balances within the U.S. pharmaceutical system, ensuring that the medicines millions rely on daily remain safe and effective.
FAQs..
What is the reason for this specific blood pressure medication recall?
The recall was initiated because testing revealed levels of a nitrosamine impurity called N-nitroso prazosin above the FDA’s acceptable safety limits. Nitrosamines are common environmental contaminants, but when present in medicines at higher-than-acceptable levels over long periods, they may increase the risk of cancer.
How can I check if my specific medication bottle is part of the recall?
First, check your prescription bottle to confirm the drug name is “Prazosin Hydrochloride” and the manufacturer is “Teva Pharmaceuticals.” Then, locate the lot number on the bottle. You can check this number against the official list of recalled lots on the FDA’s Enforcement Reports website. Your pharmacist can also assist you in verifying this information.
What is the actual cancer risk from taking the recalled medication?
The FDA has classified this as a Class II recall, meaning the probability of serious adverse health consequences is considered remote. The risk of cancer is associated with long-term exposure to the impurity above acceptable levels. The recall is a precautionary measure to prevent prolonged exposure. The agency has stated that it has not received any reports of adverse events linked to this issue.
Are other blood pressure medications also being recalled for the same reason?
This recall specifically concerns certain lots of Prazosin Hydrochloride. However, in recent years, the FDA has identified nitrosamine impurities in several other drug classes, including some versions of valsartan, losartan, and irbesartan. This is part of an ongoing, industry-wide investigation to ensure drug safety, and it highlights the importance of robust regulatory systems to detect and address such issues.